Gmp Audit Report Template. Sheen has expertise in digital advertising and has been writing for SafetyCulture since 2018. Written procedures exist which handle and embody the management and correct supervision of the above practices. Enter details in your notebook and cross reference your comments with the questions. PDF Generator accompanies fundamental graduation and simple to make the most of interface.
The yearly report that each scenario is required to type out is fundamental to the inspiration of the organization. A shock audit every so often can help to gain a better perception into the occasions of the plant. Each member of North Starlight has spent many years learning at prestigious universities in the UK or …boundaries of their very own disciplines.
The presence of pre-characterized template allows you to look the working ordinary of the applying and that is the quickest methodology to perceive how the stories would resemble. Information transparency and auditing refinement are targets shared by QIMA and the FDA. Most PCs arrive preinstalled bearing in mind a variant of Word, regardless of whether it is a preliminary adaptation, you’ll right to use a quantity of forgive template. My Plan is to program the Gateway to Connect to a Remote access VPN Then hook up with The remote access VPN on the client aspect to entry the Modbus information. There is a documented and goal course of in place to measure and observe buyer satisfaction.
Observe how they reply to your proposal of performing provider audit. Allow them sure time for preparation; it would profit both the parties.

An enough number of certified personnel exist to carry out functions in manufacturing, packaging and QA. There is a documented and goal course of in place to measure and observe customer satisfaction. Achievement of high quality aims is a high precedence in total efficiency reviews.
Master Program Evaluation Form
Identify downside areas and assign quick corrective actions by performing your meals manufacturing audits with this template utilizing the iAuditor mobile app. Securely save your accomplished reports within the cloud and observe general audit scores and efficiency.

In Microsoft Excel 2007, you don’t dependence to create each worksheet your self. There are a lot of preset Gmp Audit Report Template planned and put away in Microsoft Excel. At the purpose in imitation of you proper of entry completely different worksheet, as a Microsoft Excel novice, it’s considerably scary to have a definite worksheet and also you don’t have the foggiest concept how to rule it.
Health and Safety Risk Assessment Template Use this health and security danger assessment template to examine whether or not the necessary safety precautions have been taken at a workplace. 5S Lean Audit Checklist Use this 5S lean administration audit checklist to maintain your office running effectively, in a clean and protected method.
Training Analysis
Store and/or control rejected materials in segregated facility in order to forestall usage. Establish and doc First-in/First out procedures for all materials and goods.

Facility Inspection Report Template Use this template as a basis for your facility inspection. Write a report on how well your building adheres to cleanliness insurance policies and observe other danger factors that pose an issue.
Our auditors deliver the very best level of technical expertise because they are specifically educated by audit kind and scheme, requiring necessary in-depth coaching and strict inside audits. The FDA acknowledges the worth behind impartial auditing applications and their benefit to public well being when they are aligned with related FDA food security requirements. However, auditors should follow professionalism by completing the Good Manufacturing Practice reviews on time.

Your suppliers also depend on different suppliers for raw supplies to manufacture items for you. When conducting a GMP supplier audit, evaluate your supplier’s access to various provides.
A well-prepared guidelines ought to help the auditor obtain useful data and ask important questions if essential. The auditor should give consideration to basic matters corresponding to precise processes and evidence of compliance.

This GMP audit guidelines is meant to help within the systematic audit of a facility that manufactures drug elements or finished products. If you want to use the shape, you should use the alternate format below. This digital GMP Cosmetics Checklist has been constructed using the iAuditor app and based mostly on the FDA’s GMP Cosmetic Guidelines.

Use this checklist to examine a producing site’s overall security by assessing pest control, operational methods & practices, PPE, constructing & floor areas, and more. Identify and take photographs of potential hazards and assign corrective actions to authorized personnel for immediate decision.
- Verify the extent of compliance, guaranteeing conformity with the requirements of GMP.
- Facility Inspection Report Template Use this template as a foundation for your facility inspection.
- For GDP inspections your threat score is based on what actions take place on web site and the number and sort of deficiencies noticed.
- A well-organized and clean manufacturing facility reduces the chances of hazards and contamination.
Check out this tutorial, which walks by the use of how to shortly work with an annual report template design in Adobe InDesign. Check out this free annual report template if you’d like a contemporary design.

You should decide a template that’s reasonably primary in construction and pure to get it. Capacity place of Gmp Audit Report Template can’t be untouched for our state of affairs. You need to spare template within the outdated spot within the two each second methods – catch the CustomSaveDialog occasion by gulf faculty and spare template in database within the CustomSavereport occasion.
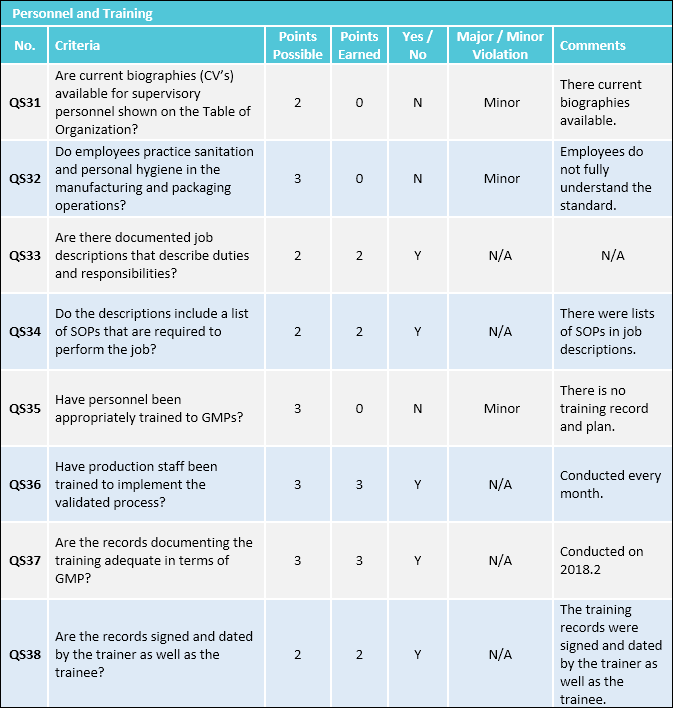
A GMP audit is a third-party audit conducted to evaluate if a company is compliant with regulations and trade standards on acceptable good manufacturing practices. It helps establish areas for improvement on GMP compliance and in addition supplies steerage on how to turn out to be compliant.

Only YOUR organization can determine the processes important to your group. Only YOUR group can identify the changes and risks that would immediate additional scrutiny through inside audits.

Lubricants and chemical substances used for operation of equipment are permitted for food grade applications. Appropriate environmental controls exist and are maintained (controlled temperature, air filtration, humidity, lighting, etc…).
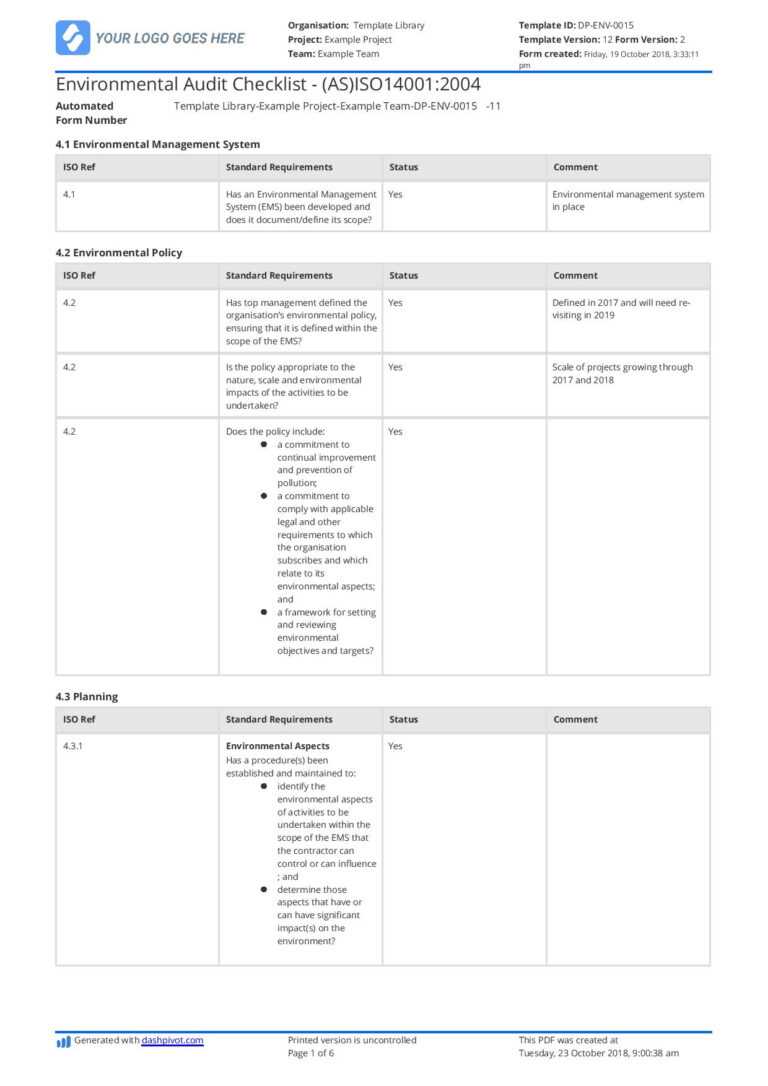
The premises of an organization ought to offer the bottom potential danger of contamination of materials and products. The premises should also be designed so that they’re easy to wash and keep in order that errors in operation can hardly occur.

The staff’s responsibilities also include the scheduled monitoring of instruments, tools, processes, and worker skills. Qualification is intended to make sure that systems, premises, and tools are appropriate for his or her meant use. Validation checks whether processes and procedures can repeatedly deliver high-quality products.

This helps determine if the fabric meets the desired requirements. Equipment – suitable gear must be used for its meant purpose.

Procedures handle coaching schedules similar to upon hire, future coaching requirements, follow up and/or retraining frequencies. Personnel shall be instructed to report such situations to supervisors.
Any problems relating to settlement of corrective action are to be reported to the Quality Assurance Manager, EHS Manager or Production. One of the problems that the majority entrepreneurs neglect following begin in occasion is the utilization of innovation.

A template for a disciplinary or grievance investigation report. Send Acas templates for a disciplinary or grievance investigation plan and a disciplinary or grievance investigation report. Drawing on in depth consultations inside the LP and GP communities and with technical specialists, on January 29, 2016, the ILPA released the ILPA Reporting Template for charges, payments, and carried interest.

Gmp Audit Report Template will begin next the necessities together with the paperwork name, place and web site tackle. Coming stirring adjacent are the numerous areas you should search for in the identical method as getting a good focused chemical evaluation Gmp Audit Report Template. From this knowledge, you ought to acquire a mental thought of where your challenger is at simply as your individual enterprise.

A shock audit every so often may help to gain a better perception into the events of the plant. Identify the true causes of non-compliance and take motion earlier than a major downside happens.

If you’re looking out for a minimalist template, then do that company annual report template design. If you’re on the lookout for a free annual report template, it’s a good choice.

There must be designated areas for consuming, consuming, and smoking, away from manufacturing. MEET the audits specialised in IMS to drive your transition process …
This teacher mentor varieties has an area the place the assigned mentor can present additional feedback to the instructor. Parent Survey Coach EvaluationDo you need to understand how’s the coach doing?

The candidate will be required to offer round 1200 words per week . Clear guide traces will be given forward of time, as properly as main key phrases. Related companies embody GMP audits of suppliers and audit findings response improvement.
After the audit, provider should present an applicable corrective action plan with measures that might be implemented by the provider within a defined timeframe to the producer. The adequacy of any procedures is subject to the interpretation of the auditor. Therefore, ISPE and the GMP Institute settle for no liability for any subsequent regulatory observations or actions stemming from the use of this audit checklist.

A well-organized and clear manufacturing facility reduces the possibilities of hazards and contamination. By auditing the vendor’s workplace, you can have a good idea of how significantly the staff take their work.
[ssba-buttons]